Antifungal assays (Minimum Inhibitory Concentration)生物自显
发布日期:2013-05-17 10:54
来源:网络
作者:admin
浏览次数:
Antifungal assays (Minimum Inhibitory Concentration)
Introduction
In its most general terms, susceptibility testing refers to the idea of mixing a fungal culture with an antifungal agent and seeing what happens. In general, we are interested in determining the lowest concentration of an antifungal agent that appears to inhibit growth (minimum inhibitory concentration, MIC) the fungus. If this level is low enough, then the drug may work against an infection. The approach to testing is different for yeasts and moulds.
Dilution methods are used to determine the MIC of antimicrobial agents and are the reference methods for antimicrobial susceptibility testing against which other methods, such as disk diffusion are calibrated. In dilution tests, microorganisms are tested for their ability to produce visible growth on a series of agar plates or in test tubes or microplate wells of broth containing dilutions of the microbial agent.
In determining the MIC values growth indicators are used and not turbidity because plant extracts are frequently turbid or causes precipitates when mixed with microbial growth media. In this project INT was used.
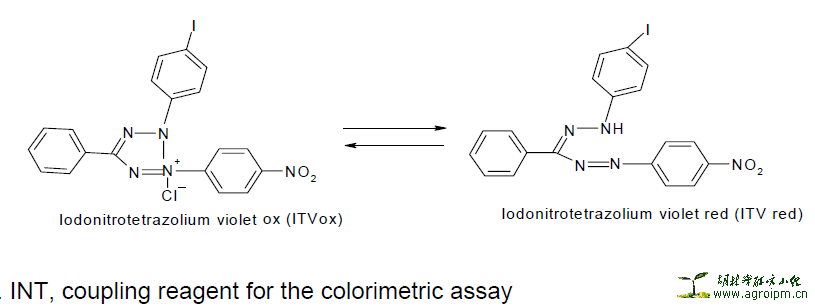
p-iodonitrotetrazolium violet (INT) Reaction
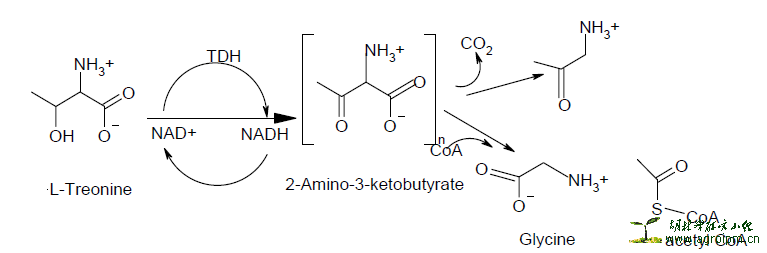
The reaction is based on the transfer of electrons from NADH, a product of the threonine dehydrogenase [TDH] catalyzed reaction, to the tetrazolium dye [p-iodonitrotetrazolium violet]. Threonine dehydrogenase [TDH] from bacteria/fungi catalyses the NAD-dependent oxidation of threonine to form 2- amino-3-ketobutyrate and NADH. During the active growth of bacteria/fungi, an electron is transferred from NADH [which is colourless in the visible range] to p-iodonitrotetrazolium violet resulting in a formazan dye, which is purple in colour.
Therefore, the clear zone(s) on the chromatogram indicate areas of inhibition [zones where no active growth of bacteria has taken place].
Materials and Method
Fungal test organisms
Five fungi were obtained from the Bacteriology Laboratory, Department of Veterinary
Tropical Diseases, Faculty of Veterinary Science and used as test organisms. These fungi represent the different morphological forms of fungi, namely yeasts (Candida albicans and Cryptococcus neoformans), thermally dimorphic fungi (Sporothrix schenckii) and moulds (Aspergillus fumigatus and Microsporum canis). They are the most common and important disease-causing fungi of animals. Candida albicans was isolated from a Gouldian finch, C. neoformans from a cheetah, and Aspergillus fumigatus from a chicken, all of which suffered from a systemic mycosis. Microsporum canis was isolated from a cat with dermatophytosis and S. schenckii from a horse with cutaneous lymphangitis. Not one of the animals had been treated prior to sampling. All fungal strains are maintained on Sabouraud dextrose agar (Oxoid, Basingstoke, UK).
Minimum inhibitory concentration
Microdilution assay
A serial microdilution assay (Eloff, 1998c) was used to determine the minimum inhibitory concentration (MIC) values for plant extracts using tetrazolium violet reduction as an indicator of growth. This method had previously been used only for antibacterial activities (Eloff, 1998c; McGaw et al., 2001). To apply it to measuring antifungal activities, a slight modification was made to suit fungal growth conditions. Residues of the different extracts were dissolved in acetone to a concentration of 10 mg/ml. The plant extracts (100 μl) were serially diluted 50% with water in 96 well microtitre plates (Eloff, 1998c). Fungal cultures were transferred into fresh Sabouraud dextrose broth, and 100 μl of this was added to each well. Amphotericin B was used as the reference antibiotic and positive control, and appropriate solvent blanks were included as negative control. As an indicator of growth, 40 μl of 0.2 mg/ml of p-iodonitrotetrazolium violet (Sigma®) (INT) dissolved in water was added to each of the microplate wells. The covered microplates were incubated for two to three days at 35 oC and 100% relative humidity. The MIC was recorded as the lowest concentration of the extract that inhibited antifungal growth after 24 and 48 hours.
The experimental design
Tests group 1: Consisted of the pathogen plus different concentrations of the extracts. This group was used to determine activity in the extract (MIC value). For each plant the following extracts were used, hexane, DCM, acetone and methanol.
Tests group 2: Positive control, it contained the pathogen plus Amphotericin B. This group was used to ensure that the pathogen was not a resistant strain and also to compare relative activities with the extracts.
Tests group 3: A pure culture containing only the pathogen. This was necessary to distinguish poor growth from inhibition and to ensure that the laboratory conditions under which the pathogens had been placed did not affect its growth.
Tests group 4: A negative control containing the pathogen together with the dissolution solvents. This ensured that the extraction solvents had no inhibitory effects on the pathogens.
Results in this chapter are represented by two papers, which are: Masoko et al., (2005) and Masoko et al., (2006).
Ref URL:http://www.aspergillus.org.uk/content/characterization-antifungal-compounds-isolated-combretum-and-terminalia-species-combretaceae





