|
作者: Mayer, AMS (Mayer, Alejandro M. S.)[ 1 ] ; Glaser, KB (Glaser, Keith B.)[ 1,2 ] ; Cuevas, C (Cuevas, Carmen)[ 3 ] ; Jacobs, RS (Jacobs, Robert S.); Kem, W (Kem, William)[ 5 ] ; |
|
来源出版物: |
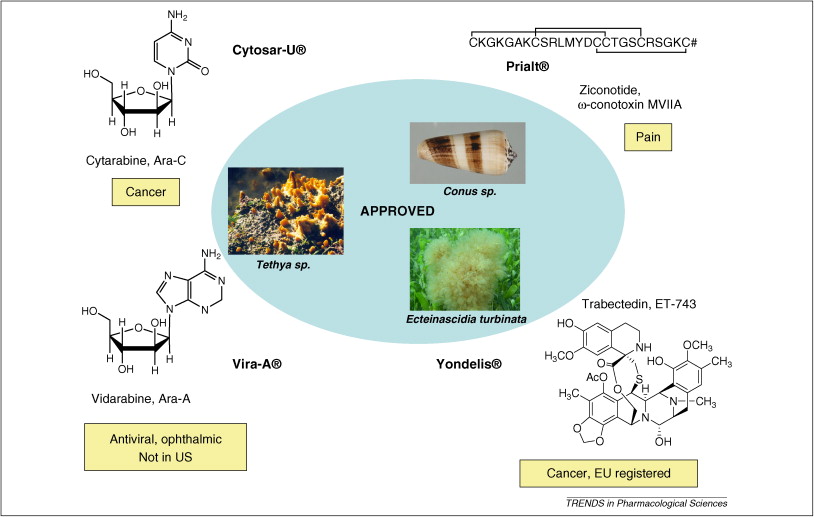
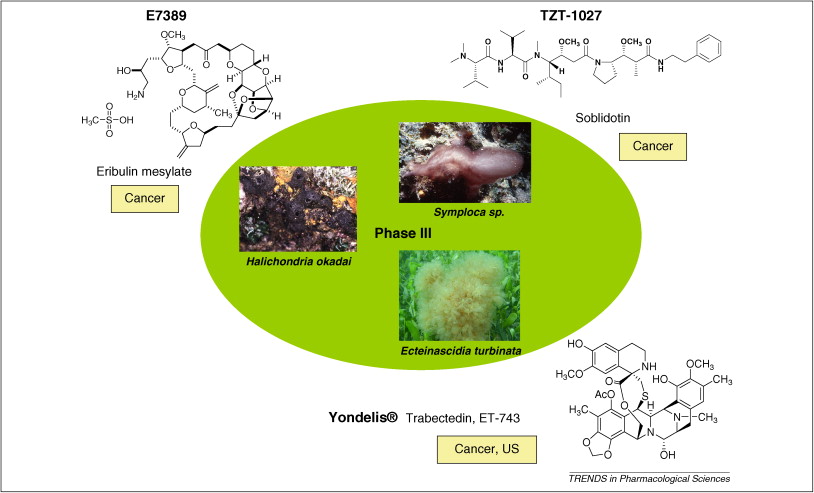
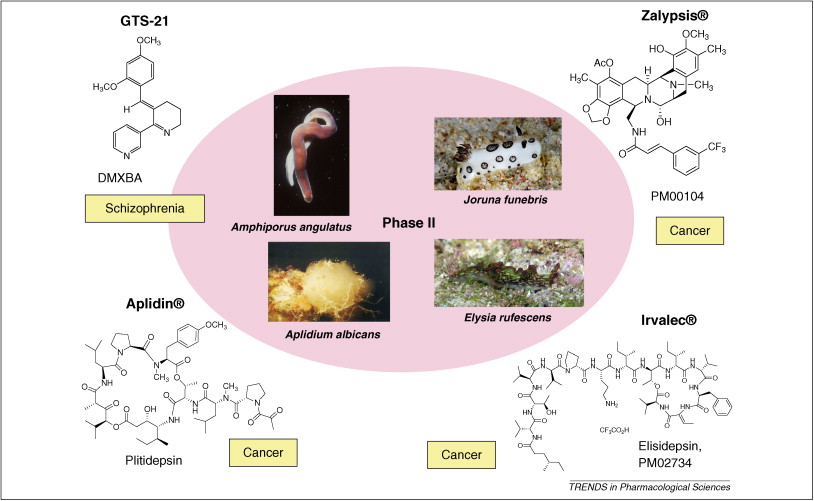
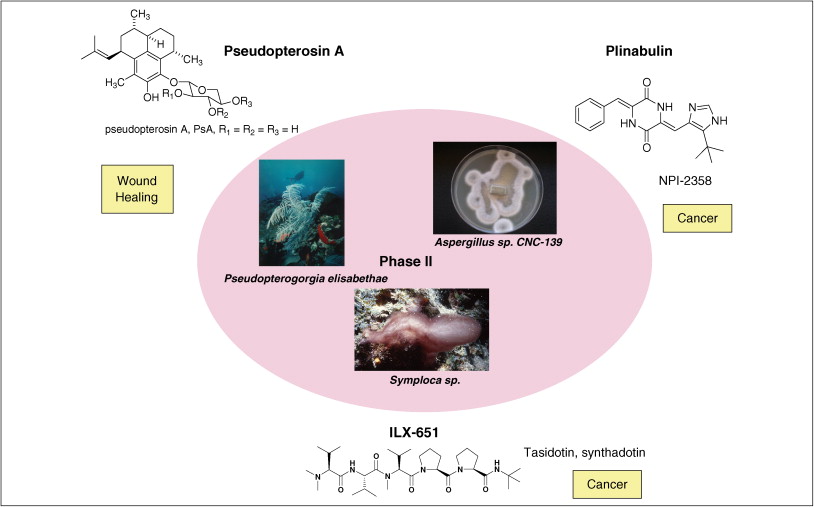
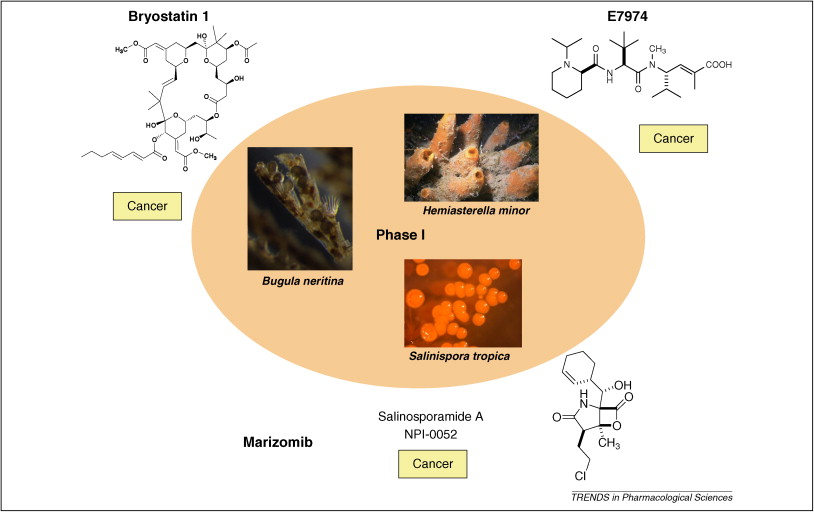
| 摘要: The global marine pharmaceutical pipeline consists of three Food and Drug Administration (FDA) approved drugs, one EU registered drug, 13 natural products (or derivatives thereof) in different phases of the clinical pipeline and a large number of marine chemicals in the preclinical pipeline. In the United States there are three FDA approved marine-derived drugs, namely cytarabine (Cytosar-U (R), Depocyt (R)), vidarabine (Vira-A (R)) and ziconotide (Prialt (R)). The current clinical pipeline includes 13 marine-derived compounds that are either in Phase I, Phase II or Phase III clinical trials. Several key Phase III studies are ongoing and there are seven marine-derived compounds now in Phase II trials. The preclinical pipeline continues to supply several hundred novel marine compounds every year and those continue to feed the clinical pipeline with potentially valuable compounds. From a global perspective the marine pharmaceutical pipeline remains very active, and now has sufficient momentum to deliver several additional compounds to the marketplace in the near future; this review provides a current view of the pipeline. |
 |
The odyssey of marine pharmaceuticals: a current pipeline perspective |

以下内容转载自小木虫 哥特复兴VS [1]Han-Dong Sun,Yi-Ming Shi:LCUV-Guided Isolation and Structure Determination of Lancolide E: A Nortriterpenoid with a Tetracyclo [...

同一种微生物在不同的国家或地区常有不同的名称,这就是俗名。俗名在局部地区可以使用,但不便于交流,容易引起混乱。为在世界范围...

人是能够思想的苇草 帕斯卡尔 人只不过是一根苇草,是自然界最脆弱的东西;但他是一根能思想的苇草。用不着整个宇宙都拿起武器来才能...

1 目的 规范TENSOR 27红外光谱仪的操作程序,正确使用仪器,保证设备安全和检测的顺利进行。 2 使用环境 电源电压:85~265V,47~65Hz 温度范...