Revisiting the Polyol Synthesis of Silver Nanostructures: Ro
发布日期:2023-11-09 09:58 浏览次数:
Revisiting the Polyol Synthesis of Silver Nanostructures: Role of Chloride in Nanocube Formation
-
Zhifeng Chen
-
,
-
Tonnam Balankura
-
,
-
Kristen A. Fichthorn*
-
, and
-
Robert M. Rioux*
-
Cite this: ACS Nano 2019, 13, 2
Publication Date:January 23, 2019
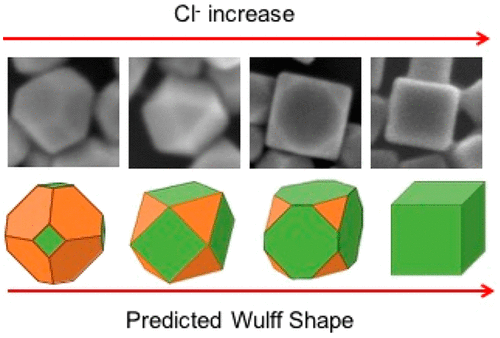
Chloride (Cl–) is often used together with polyvinylpyrrolidone (PVP) in the polyol synthesis of Ag nanocubes. In the literature, shape control is attributed predominantly to the preferential binding of PVP to Ag(100) facets compared to Ag(111) facets, whereas the role of Cl– has not been well studied. Several hypotheses have been proposed regarding the role of Cl–; however, there is still no consensus regarding the exact influence of Cl– in the shape-controlled synthesis of Ag nanocubes. To examine the influence of Cl–, we undertook a joint theoretical–experimental study. Experimentally, we examined the influence of Cl– concentration on the shape of Ag nanoparticles (NPs) at constant H+ concentration. In the presence of H+, in situ formed HNO3 etches the initially formed Ag seeds and slows down the overall reduction of Ag+, which promotes the formation of monodisperse Ag NPs. Ex situ experiments probed the evolution of Cl– during the growth of Ag nanocubes, which involves the initial formation of AgCl nanocubes, and their subsequent dissolution to release Cl–, which adsorbs onto the surfaces of single crystal seeds to impact shape evolution through apparent thermodynamic control. The formation of cubes is independent of the source of AgCl, indicating temporal control of the Cl– chemical potential in solution leads to high-yield synthesis of Ag nanocubes. Increasing the concentration of Cl– alone leads to a progression in shape from truncated octahedra, to cuboctahedra, truncated cubes, and ultimately cubes, directly demonstrating the importance of Cl– in Ag NP shape control. We used ab initio thermodynamics calculations based on density functional theory to probe the role of Cl– in directing shape control. With increasing Cl chemical potential (surface coverage), calculated surface energies γ of Ag facets transition from γ111 < γ100 to γ100 < γ111 and predict Wulff shapes terminated with an increasing (100) contribution, consistent with experimental observations. The combination of theory and experiment is beneficial for advancing the understanding of nanocrystal formation.