The odyssey of marine pharmaceuticals: a current pipeline pe
发布日期:2013-01-15 10:12 浏览次数:
|
作者: Mayer, AMS (Mayer, Alejandro M. S.)[ 1 ] ; Glaser, KB (Glaser, Keith B.)[ 1,2 ] ; Cuevas, C (Cuevas, Carmen)[ 3 ] ; Jacobs, RS (Jacobs, Robert S.); Kem, W (Kem, William)[ 5 ] ; Little, RD (Little, R. Daniel)[ 4 ] ; McIntosh, JM (McIntosh, J. Michael)[ 6 ] ; Newman, DJ (Newman, David J.)[ 7 ] ; Potts, BC (Potts, Barbara C.)[ 8 ] ;Shuster, DE (Shuster, Dale E.)[ 9 ] |
|
来源出版物: TRENDS IN PHARMACOLOGICAL SCIENCES 卷: 31 期: 6 页: 255-265 DOI: 10.1016/j.tips.2010.02.005 出版年: JUN 2010 |
|
|
|
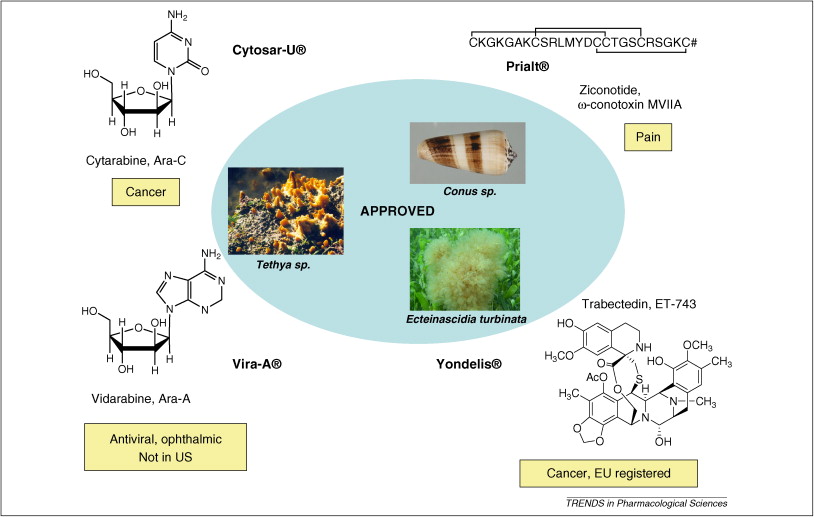
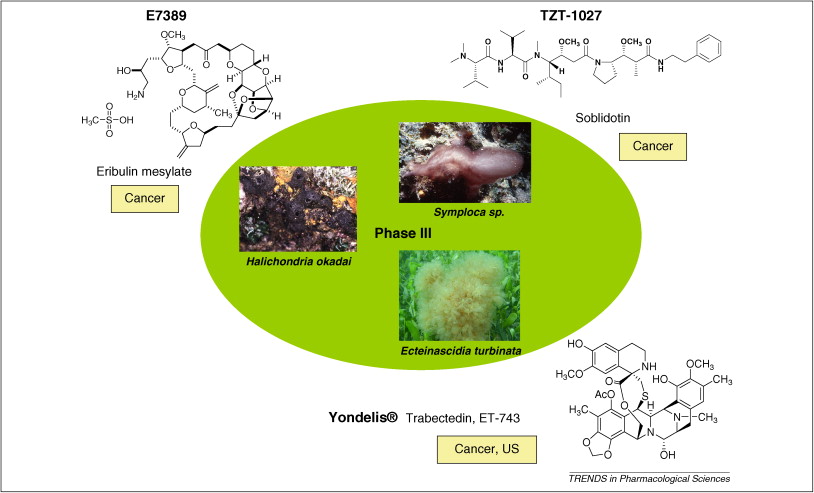
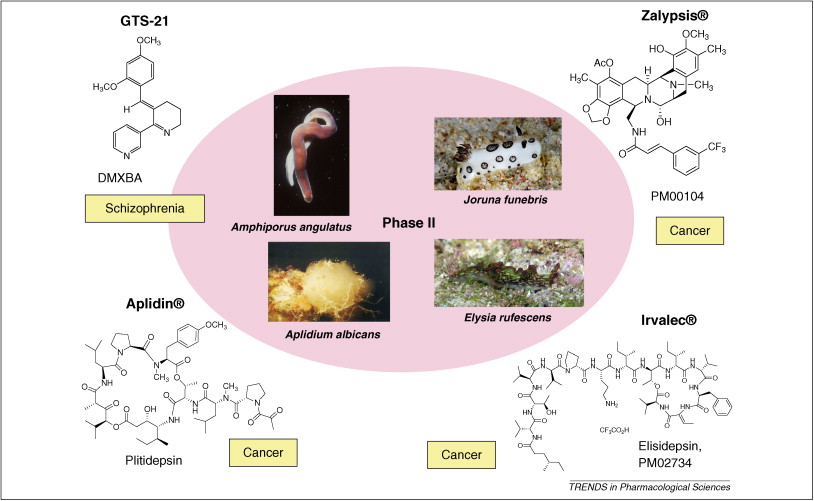
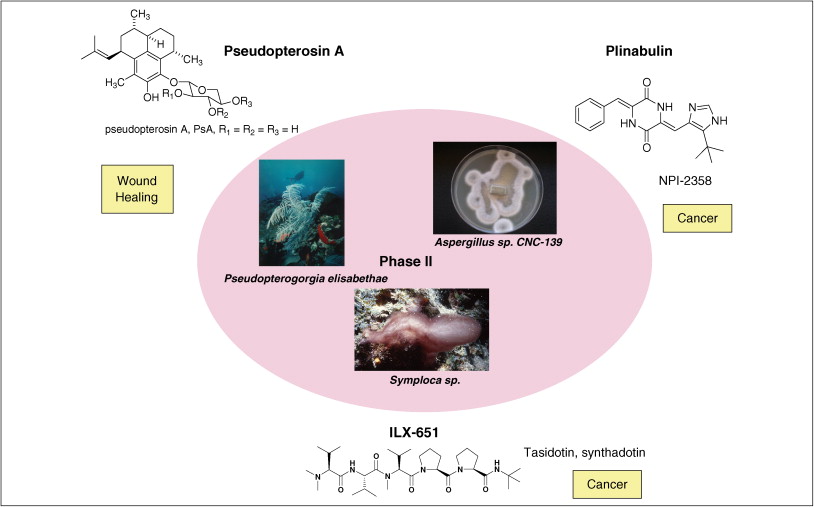
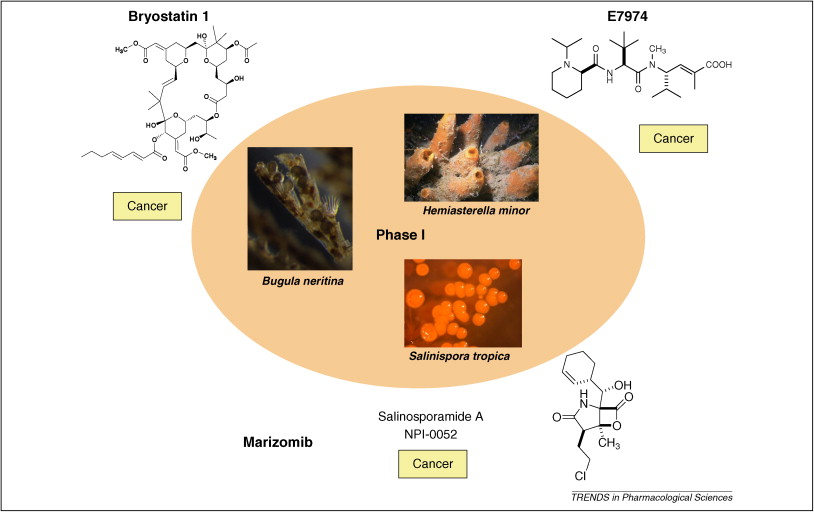
摘要: The global marine pharmaceutical pipeline consists of three Food and Drug Administration (FDA) approved drugs, one EU registered drug, 13 natural products (or derivatives thereof) in different phases of the clinical pipeline and a large number of marine chemicals in the preclinical pipeline. In the United States there are three FDA approved marine-derived drugs, namely cytarabine (Cytosar-U (R), Depocyt (R)), vidarabine (Vira-A (R)) and ziconotide (Prialt (R)). The current clinical pipeline includes 13 marine-derived compounds that are either in Phase I, Phase II or Phase III clinical trials. Several key Phase III studies are ongoing and there are seven marine-derived compounds now in Phase II trials. The preclinical pipeline continues to supply several hundred novel marine compounds every year and those continue to feed the clinical pipeline with potentially valuable compounds. From a global perspective the marine pharmaceutical pipeline remains very active, and now has sufficient momentum to deliver several additional compounds to the marketplace in the near future; this review provides a current view of the pipeline. |