发布日期:2012-12-19 17:49 浏览次数:
![]()
The reaction of triphenylphosphine and tetrahalomethanes (CCl4, CBr4) with alcohols is a ready method to convert an alcohol to the corresponding alkyl halide under mild conditions. The yields are normally high.
This reaction is somewhat similar to the Mitsunobu Reaction, where the combination of a phosphine, a diazo compound as a coupling reagent, and a nucleophile are used to invert the stereochemistry of an alcohol or displace it.
The reaction proceeds by activation of the triphenylphosphine by reaction with the tetrahalomethane, followed by attack of the alcohol oxygen at phosphorus to generate an oxyphosphonium intermediate. The oxygen is then transformed into a leaving group, and an SN2 displacement by halide takes place, proceeding with inversion of configuration if the carbon is asymmetric.
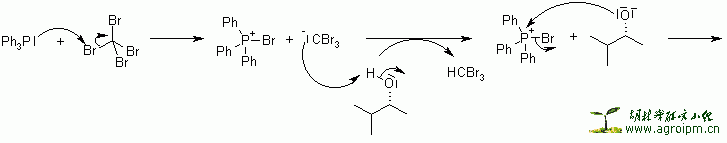
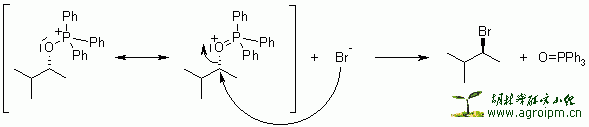
Recent Literature
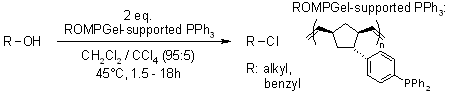
ROMPgel-Supported Triphenylphosphine with Potential Application in Parallel Synthesis
E. Arstad, A. G. M. Barrett, B. T. Hopkins, J. Koebberling, Org. Lett., 2002, 1975-1977.
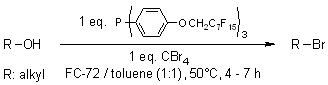
Conversion of alcohols to bromides using a fluorous phosphine
L. Desmaris, N. Percina, L. Cottier, D. Sinou, Tetrahedron Lett., 2003, 44, 7589-7591.
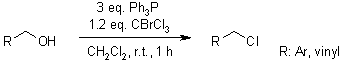
Stoichiometric bromotrichloromethane in acetonitrile can replace solvent quantities of carbon tetrachloride in the synthesis of gem-dichloroalkenes from aldehydes in the presence of triphenylphosphine. Similarly, bromotrichloromethane in dichloromethane can be used for the Appel reaction of benzyl alcohols to form benzyl chlorides at r.t., which is commonly carried out in refluxing carbon tetrachloride.
S. G. Newman, C. S. Bryan, D. Perez, M. Lautens, Synthesis, 2011, 342-346.
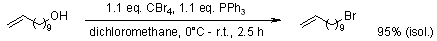
The facile preparation of alkenyl metathesis synthons
T. W. Baughman, J. C. Sworen, K. B. Wagener, Tetrahedron, 2004, 60, 10943-10948.
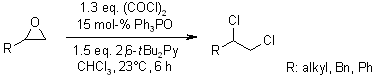
Catalysis of Phosphorus(V)-Mediated Transformations: Dichlorination Reactions of Epoxides Under Appel Conditions
R. M. Denton, X. Tang, A. Przeslak, Org. Lett., 2010, 12, 4678-4681.
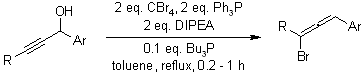
Chemoselective Isomerization of Secondary-Type Propargylic Alcohols to Propargylic/Allenic Bromides, and Brominated Dienes with Appel-Type Reaction Conditions
N. Sakai, T. Maruyama, T. Konakahara, Synlett, 2009, 2105-2106.